October 6th Program -- Fluid Inclusions: Happy Little Accidents of the Mineralogical World, presented by Dr. Joshua Field
by Andy Thompson, MSDC Secretary
Dr. Joshua Field completed his doctorate in geology in 2017 and has been sharing his knowledge and expertise both in academia and industry. His presentation to MSDC drew on his analytic work with inclusions. They are the “little accidents” that often occur when solid crystals precipitate out of a fluid during or after the formation of the crystal.
The “happy” aspect of those trapped fluid inclusions is they allow researchers to discover aspects of the environment in which the crystals were formed and sometimes provide hints as to how the crystal developed over time.
“Because most mineral collectors are not familiar with the analysis of fluid inclusions,” Joshua said, his talk would “be going over the basics of fluid inclusion” which he did with clarity and in three parts as listed below: the basics, the science, and showing examples of inclusions in minerals and research with inclusions.

Part One: The Basics of Inclusions
Simply put, crystals are formed by precipitating out of fluids. But sometimes, the process is not perfect and a bit of the original fluid does not crystalize and instead becomes trapped as fluid or vapor which is engulfed within the growing crystal. At other times, a small part of the crystal melts and becomes a liquid trapped within the crystal. But that happenstance will not be addressed in this program.
Joshua showed a short video illustrating the imperfect ways in which crystals form. It showed a halite crystal, parts of which grew in different directions and rates. It also had some rough surfaces, all of which provided nooks and crannies in which fluid could be trapped. He showed many examples of inclusions, most of which were at the micro size, so not easily visible to the naked eye.
Inclusions are important, in part because they can provide researchers with valuable information about the temperature, pressure and fluid’s chemical composition at the time of the crystal formation. What they can not tell us, however, is the exact time when the crystals were formed.
Part Two: The Science of Inclusions
The bulk of Joshua’s presentation focused on the specific types of inclusions found in crystals and what information researchers can discover about the context of the inclusions’ formation. He emphasized that the value of the analysis comes from the information gathered from a cluster of crystals, a population, rather than from findings pertaining to an individual crystal. That being said, based on how the inclusions were formed, there are three types of inclusions described and illustrated below.
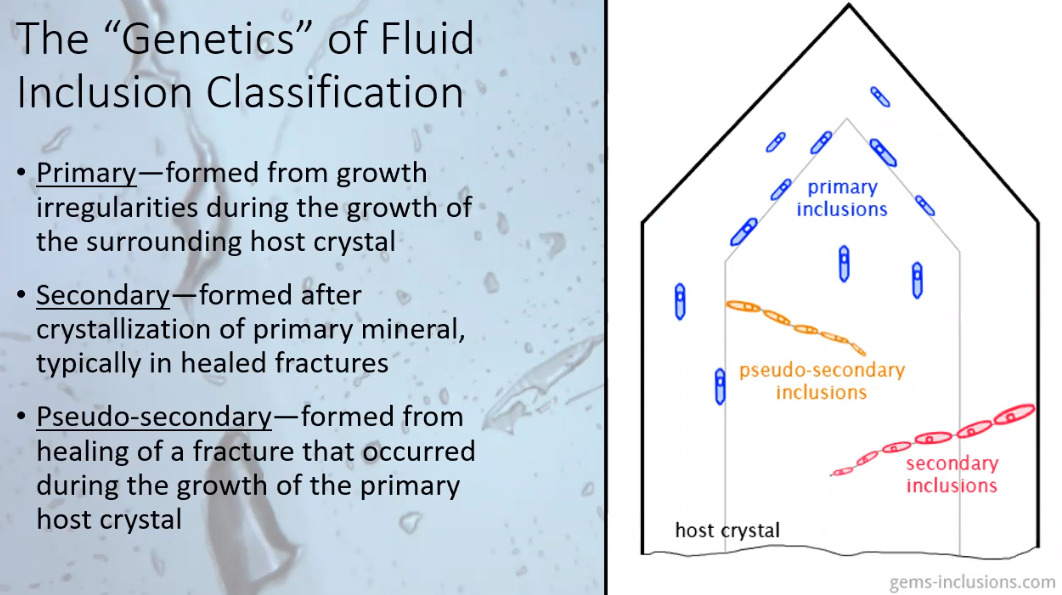
1. The primary inclusions form along the growth zones of the crystal.
2. The secondary inclusions happen after the crystal growth has been completed. They do not form along the crystal’s growth zones but rather along a plane which cuts across the growth zones.
3. The third type of inclusions, “pseudo-secondary inclusions,” form when there has been a hiatus in the crystal growth or there has been a fracture in the crystal and fluid seeps into that fracture and is captured inside. Also, this third type of inclusion always terminates at a growth zone boundary and never ends at the outside edge of the host crystal.
Joshua said that inclusions become particularly interesting when researchers determine the content of the inclusions which can change over time. He noted fluid inclusions have four typical forms of content or phases listed below.

Mineral collectors are curious about how researchers can learn about the environment in which the minerals and inclusions formed once they identify the content of the fluid inclusion. One method is for researchers to heat up and cool down the crystals and inclusions and watch any changes that occur. By determining the temperature at which the inclusion melts, that helps identify the pressure at the time the inclusion was trapped.
Joshua said that for the MSDC presentation, he preferred to avoid describing the complicated analytic method. He thought it sufficed to say that researchers use previously calculated temperature vs. pressure and temperature vs. NaCl weight charts, derived from earlier studies, to gain valuable information as to the environment in which the researchers’ inclusions were formed.
“The reason we care about any of this this is that getting the salinity and homogenization temperature can point you in the direction of what type of deposit you are looking at.” As examples, he cited deposits of volcanogenic massive sulfide; epithermal; intrusion-related base metal; porphyry copper; Mississippi Valley-Type; and skarn.
Part Three: Examples and Research
The final part of Joshua’s presentation gave some examples of how inclusion research makes a difference. As an example, he drew from his doctoral dissertation which worked on analyzing samples of trace inclusions within sphalerite crystals in selected regions of the continental U.S. Joshua asked why, for example, despite finding gram-size quantities prevalent throughout the Upper Mississippi Valley, in only a few locations did the zinc-lead deposits amount to millions of tons.
In the photo below, showing one the Mississippi Valley rock formation sites which Joshua explored, he found a tiny sample of sphalerite on limestone identified within the blue circle in the photo. Joshua wanted to know, given all relevant contributing elements being equal, such as abundant conduits for fluid flow, what factors were associated with the respective formation of large or tiny quantities of zinc deposits.
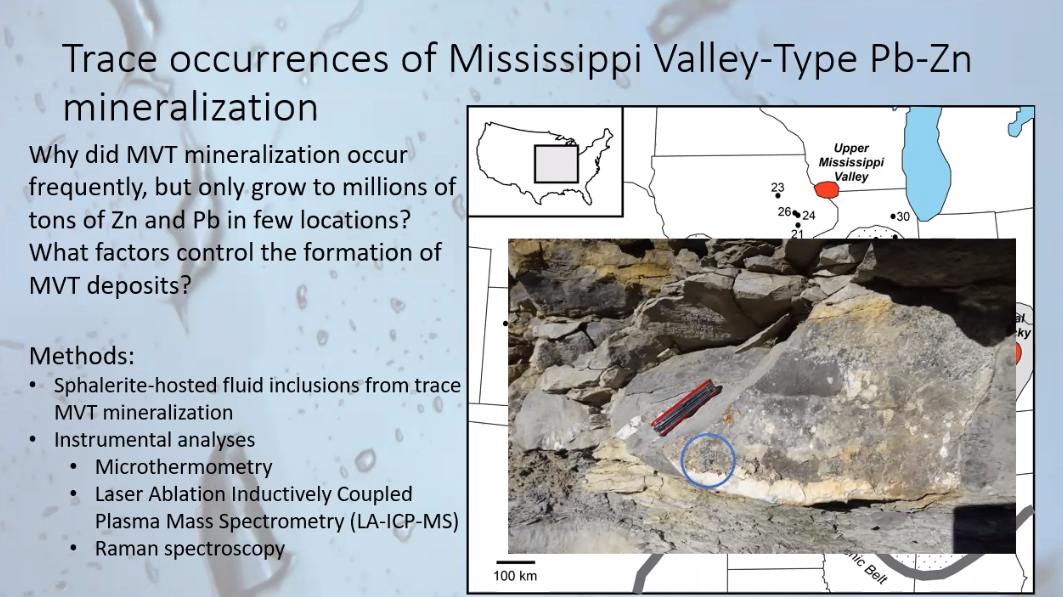
Briefly stated, Joshua used analysis of the fluid inclusions of his samples, their primary, secondary and pseudo-secondary types of inclusions, along with high-tech instruments noted in the above slide as “Methods,” to help explain the genesis of the very large and tiny zinc deposits.
When Joshua analyzed measurements from over 250 inclusions, he found that the amount of zinc metal in the fluid was not associated with variations in the size of the deposits, whether tiny or huge, found at their respective sites. Rather, it was the presence of reduced sulfur and methane that was associated with the massive deposits of zinc. He noted that there are several ways of assessing the diverse sites to predict the extent of their metal ore deposits. Analysis of their fluid inclusions, however, was the focus for his dissertation research.
Examples of Fluid Inclusions
Joshua concluded his presentation by showing examples of the fluid inclusions with which he worked. He used laser analysis to determine which metals were trapped within the specimen. He found that in 60% to 70% of the massive deposits of zinc or lead metal, it was the presence of sulfur or reduced sulfur in the fluid inclusions which was associated with massive metal deposits, not the amount of zinc contained in the fluid inclusions.
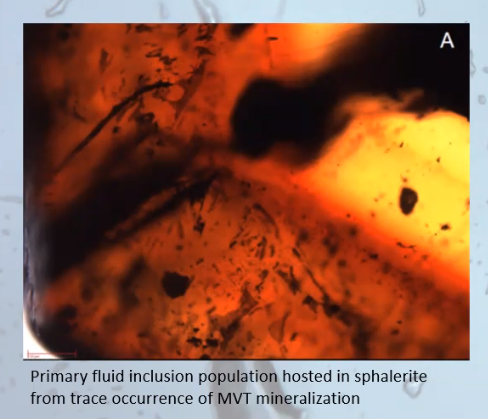
Below is a sphalerite specimen which contains a primary fluid inclusion shown as a line of tiny black dots that stream in the orange line from about the 4 o’clock position on the right side of the photo, slightly rising toward the center of the specimen.
As an example of other research with inclusions, Joshua shared a study which he found particularly interesting, relating to the current explorations of extremophile microorganisms. The researchers examined algae which they found to have been trapped within halite crystals for about 35,000 years. When the fluid inclusions containing the algae were removed from their crystals and nurtured, a number of the samples were found to be viable, akin to Jurassic Park.
Joshua then shared with his MSDC audience, from his personal collection, a number of inclusion specimens shown below. He pointed out the peculiarities he found most interesting in each.

His is favorite specimen, shown below, is a beautiful translucent spodumene he obtained from Pakistan. He pointed out a tiny bubble trapped in fluid at the top center, the bubble inclusion clearly moved as he tilted the mineral from side to side.

Another of his favorites, shown below, is a beryl specimen which contains four or five microscale bubbles each enclosed in its own crystal chamber. He identified all of the inclusions as “primary” because they all seem to have formed along the rough edge of an interior circle in the core of the specimen. All the bubbles are the same size and all moved when the specimen was tilted.
What these last specimens represent for Joshua is “they combine my love of geology and visually are very impactful.”

Conclusion and Follow Up Questions
Yury and Dave thanked Joshua for his wonderful presentation. Joshua then invited questions from the attendees. Dave raised the question of proper terminology. In effect, he asked if the term “in hydro” which he finds many collectors and dealers use to apply to all inclusions, is accurate. Joshua indicated that such a use is confusing. The term “in hydro” is typically used to refer to water inclusions in larger specimens such as agates and geodes. But as his program demonstrated, most of his micro inclusions were not water but rather contained a more complex chemistry.
Additionally, Beth H raised the question of whether, after heating and cooling the crystals as part of the analyses and discovery process, the inclusions returned to the same temperature as before those processes started. “They usually do,” Joshua responded and he provided additional information noting that researchers typically do the heating first, rather than the cooling, which could potentially damage or alter the crystal structures due to possible formation of ice crystals.
Quintin raised the question about the identity of the liquid found in rocks, such as those of the Aris quarries in Namibia, in which fluid, he said, is abundant. Joshua said he was not familiar with those particular Namibian rock formations. Joshua speculated that the Namibia rock fluid may well be similar to the fluid that is often found in geodes. Quintin noted that he believed the water found in such Namibian rock probably had been there from the beginning of the rock formations themselves, rather than from subsequent fractures which were then filled in with fluids.
Yury asked for and received a clarification as to the importance of distinguishing between primary and secondary inclusions. Is it mainly for the purpose of determining the timing or when the minerals were formed? Joshua suggested the value of the distinction between primary and secondary inclusions depends on how researchers use that distinction to answer their own questions. Joshua, for example, used the distinction to determine what factors influenced the size of the metal deposits, whether miniscule or hundreds of tons of metals, as explained above. For Joshua’s research, he said he was not interested in what happened to the secondary inclusions after the rock formation and metal deposit had been made. For that reason, he studied the primary inclusions, and the presence or absence of sulfur and methane which were present when the very large quantities of metals were originally deposited.
On the other hand, if a researcher were interested in studying the effects of a tectonic event, then studying the secondary inclusions would become central. Quakes would cause cracking in the mineral crystals and be the occasion of the formation of extensive secondary inclusions.
Jeff told the interesting story of how back in 1980, the U.S. government was searching for a safe place to store its nuclear waste. He was a summer intern and their project examined the mineral inclusions found in the salt dome caverns along the U.S. Gulf coast. When they learned that those inclusions contained tiny amounts of water, and that the water migrated toward heat, that ended the investigation of those extensive salt caverns as a possible storage place for hot nuclear waste.
Kathy and Joshua then told stories of finding various inclusions in Herkimer diamonds and she encouraged everyone to take a second look at their mineral collection with that in mind.
As part of the meeting’s Show and Tell portion, Dave invited attendees to share any minerals they brought to the event. At the end of the evening, he again thanked our presenter and everyone for attending and participating in the October meeting.
