A Very Close Look at Rodingite from the Hunting Hill Quarry, Maryland
Synopsis of the November Program presented by Tim Rose
by Andy Thompson, MSDC Secretary

Cindy Schmidtlein, MSDC’s Vice President for Programs, introduced geologist Tim Rose as our club’s sponsor within the Smithsonian National Museum of Natural History and manager of its analytical labs. Tim grew up in Montgomery County, not far from the Hunting Hill Quarry. A challenge for young Tim and his friends was that the quarry was off limits to children. So, “we would collect rocks anywhere we could,” including the beautiful mixed colored green-brown-grey rocks (rodingite) and smaller deep green crystals (diopside) sometimes found mixed in with the crushed darker gray-black stones in local parking lots.
Little did the budding child-geologist know that about that same time he and friends were collecting rocks, an adult USGS geologist, Dr. David Larrabee, did have access to the quarry and was collecting and studying the same kind of colorful rocks, including the highly reflective green crystals. Dr. Larrabee’s research resulted in USGS Bulletin 1283, published in 1969, illustrated below.

Many decades later, the focus of that bulletin, the rodingite of Hunting Hill Quarry, became the subject matter for the now-adult geologist Tim Rose’s presentation to the November meeting of the Mineralogical Society of DC.
As the Manager of the Department of Mineral Sciences’ Analytical Labs within the National Museum of Natural History, Tim analyzed rodingite using research tools that were not readily available to earlier generations of scientists, including David Larrabee. So that raised the question: What did Tim discover and how did it compare to the findings of David’s Bulletin 1283?
But first, an overview of the quarry provides a helpful context for understanding these research findings.
Overview of the Hunting Hill Quarry
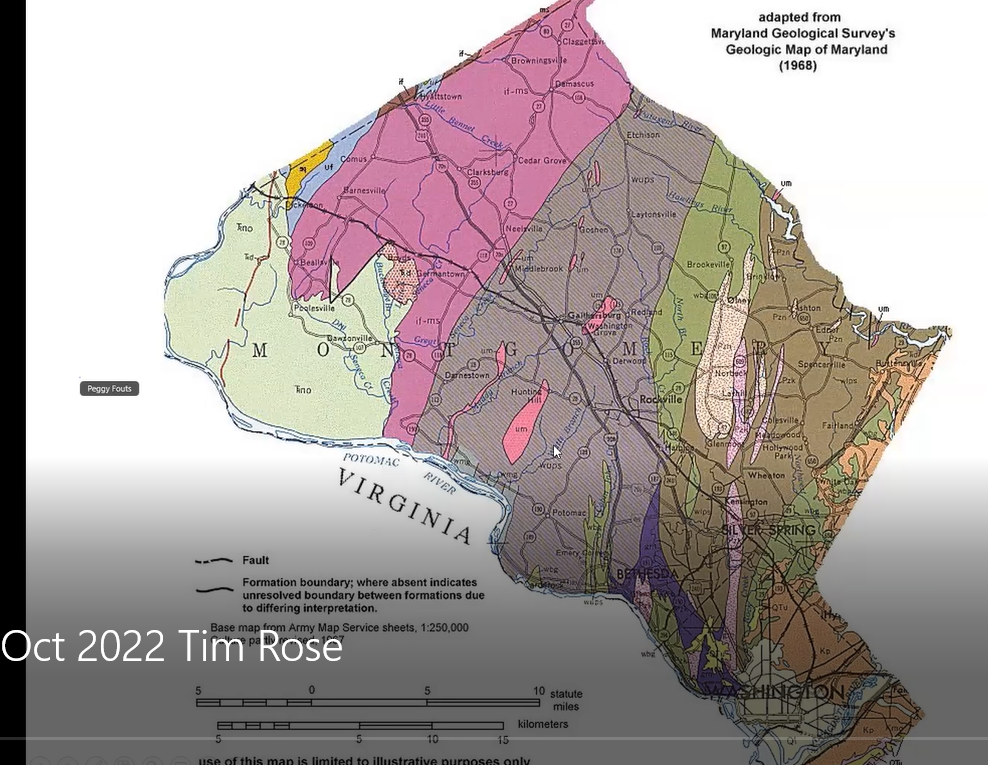
The gray Wissahickon schist area and red areas on the left side (west) of the map, Tim said, are parts of the Piedmont in Montgomery County, Maryland, in which the Hunting Hill Quarry is located. Shown below in greater detail below, the quarry is illustrated as the bright pink vertical, elliptical-shaped figure. He pointed out the tiny “UM” designation within that pink section, which identifies the quarry’s stone as “ultra mafic serpentinite,” meaning the area contains large quantities of iron and magnesium minerals.
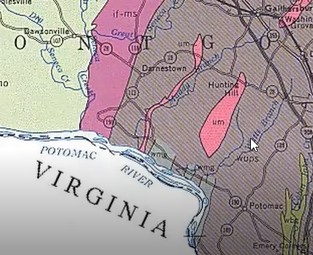
The quarry minerals were once part of the Earth’s mantle, but over millennia they underwent metamorphic changes. Having been subjected to high pressures and “squeezed” to the point that they chemically changed, they are now diverse metamorphic serpentine rocks, which for decades were mined to serve as crushed stone for concrete and large rip-rap boulders. Pictured below, the presence of the quarry is consistent with the area’s name, Rockville, Maryland.
“Rodingite” was the primary focus of Tim’s talk. He began by providing a very helpful context which included the geological structure of the quarry and an overview of its more interesting rocks and minerals. About 60 mineral specimens have been identified. Tim described rodingite as green and brown rocks, characterized as a rock comprised mainly of green diopside, brown grossular garnet and pink clinozoisite. Sometimes it also contained smaller amount of other minerals.
Structural Geology of the Quarry
The question many collectors raise when they enter a quarry is “Where are the goodies?” Tim answered that question by providing a context.
Long ago, magma intruded the ultramafic rocks deep in the earth, forming dikes of gabbro. These dikes have now become rodingite. The photo and illustration below shows these dikes as they were when Larrabee conducted his study. Tim pointed out that the rodingite dikes, shown below as white vertical streaks, have occasional cavities and calcite veins that are the best sites for collecting minerals in these rocks.

Mineralogy of the Quarry
“Most of the rocks found at the Hunting Hill Quarry are serpentine,” having the feel and green color of serpents. Tim showed a number of examples of serpentine minerals including picrolite, pictured below.

The picrolite has horizontal lines which show a form of columnar or coarsely fibrous material which is not asbestos. But other rocks contain a small amount of fibrous chrysotile asbestos which historically proved to be very problematic for human health.
Tim explained that for many years, crushed rock from this quarry had been used in roadbeds and parking lots throughout Montgomery County. After many years of this practice, a school teacher reported that throughout the County, there was asbestos in the crushed stone from Hunting Hill quarry. That disclosure precipitated a much-needed massive program for its removal and replacement. Subsequently, Tim said, the crushed serpentine had a more limited and controlled use, such as for concrete and for its ease of use as underlayment for patios.
Another specimen Tim personally collected many years ago was prehnite, the brown mineral shown below, having artistic white sprays of aragonite on its surface.

Less-frequently, collectors found the mineral coalingite, shown below, which has a bronzy or golden-brown appearance and is rich in magnesium and iron.

But, as Tim said, “the big cheese for many collectors at this quarry has always been grossular garnets,” an example of which is shown below. They are typically cinnamon brown, gemmy crystals which vary in size and are found in clumps or as individual crystals. The crystals are often within holes, or vugs, in the rodingite dikes illustrated above.

Rodingite, the Theme of the Presentation
Having given an overview of the structure and some of its interesting minerals of the Hunting Hill Quarry, Tim then focused on the main theme for his presentation, the rock rodingite. Its name comes from where it was first identified, near the New Zealand’s Roding River.
Tim explained that rodingite forms from the alteration of the original gabbro rock by the release of high pH (meaning more base than acid) water-rich fluids and calcium during the serpentinization of ultramafic rocks. So, it is clearly a metamorphic rock. Importantly, rodingite is “primarily made of grossular garnet (cinnamon), diopside (green), zoisite, clinozoisite (pink), idocrase and prehnite.” He added, this rock can appear to be similar to but should not to be confused with unakite.
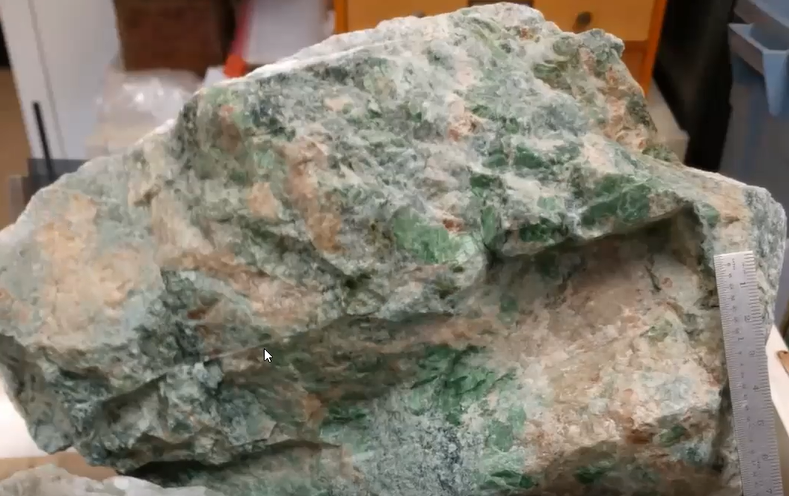
Exploring the Composition of Diopside
Tim then focused his presentation even more sharply on one of the three main minerals found in rodingite, namely the deep green and platy diopside shown below.

Remember, this smaller crystal is one Tim collected in the crushed stone of parking lots. He grew up knowing this mineral as “chrome diopside,” the name consistent with the above referenced USGS 1969 research bulletin written by Dr. David Larrabee. Tim called attention to the effect of a bright sheen reflecting off the green crystals when they are turned slightly and catch the light.
But what is the chemical composition of this diopside? Larrabee’s research identified it as follows.
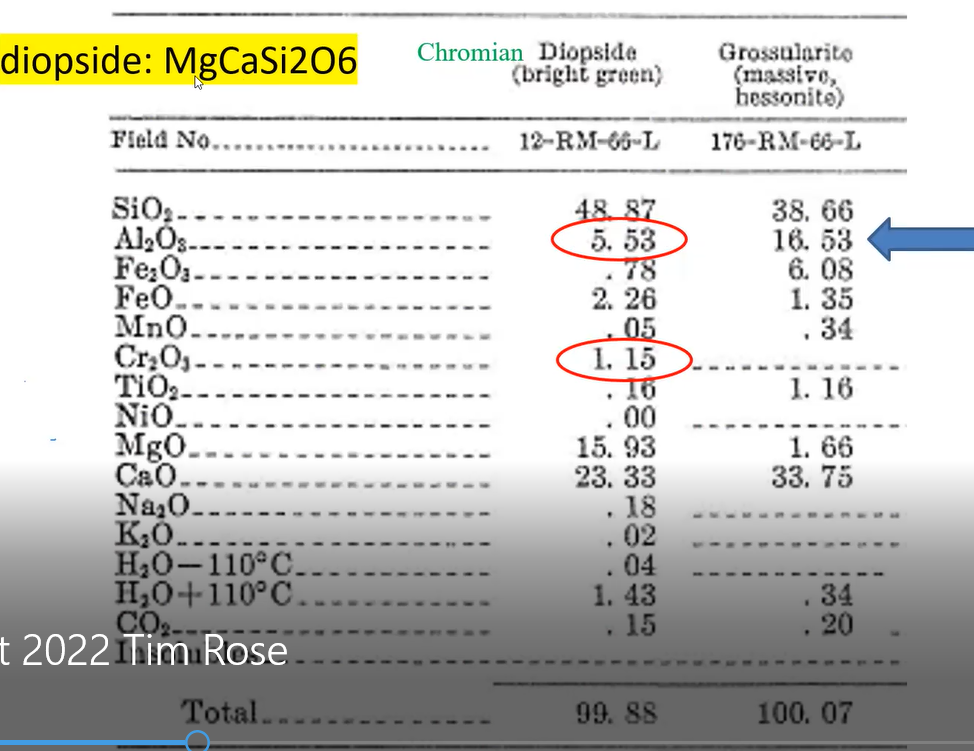
Tim noted that he highlighted in yellow the chemical formula of diopside written at the top of the slide. He pointed out that the formula does not include chromium (Cr). But because the Cr is found in the above listed contents as one of the minerals, constituting 1.15%, Tim said he added that chemical element’s name “chromium," highlighted in green, in front of the chemical formula for “diopside.” That explained why Dr. Larrabee referred to the mineral as “chromium diopside” and why Tim grew up using that name to identify the green crystals he collected.
Also, there was an immediate and practical reason for Tim’s recent analysis of this diopside mineral specimen. The NMNH was hosting an event for a congressional gathering, including children, and the staff were giving out samples of this “beautiful local mineral.” Tim saw the need to make sure the staff were confident in their knowledge of the mineral and that the displays correctly identified what they were talking about as they were giving out samples to the children and adult visitors who were coming to the museum.
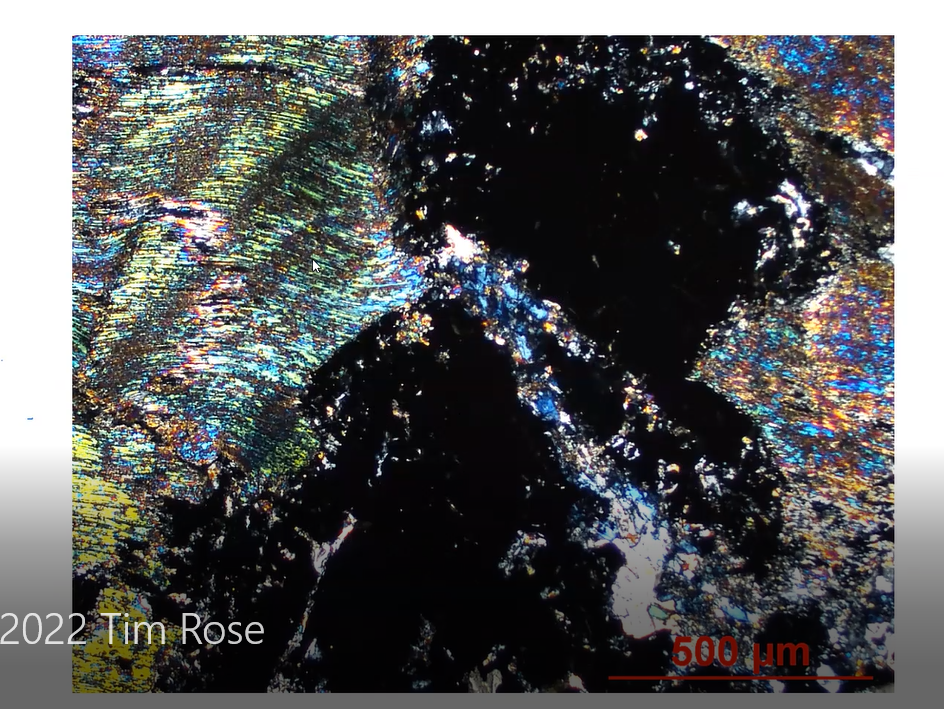
So, in order to be sure, a further analyses and exploration of the specimen’s identity was in order. As Manager of the Analytical Labs, Tim placed the petrographic thin section of the diopside specimen under a microscope and captured the image of the thin section, shown below. It is a mere 2 mm wide from left to right. When illuminated by filtered polarized light, the image shows dark sections throughout the slide. Tim identified those dark areas as the grossular garnet which, due to the slide’s thinness, shows up as black. The colorful wavy interference lines on the left are other minerals such as diopside.
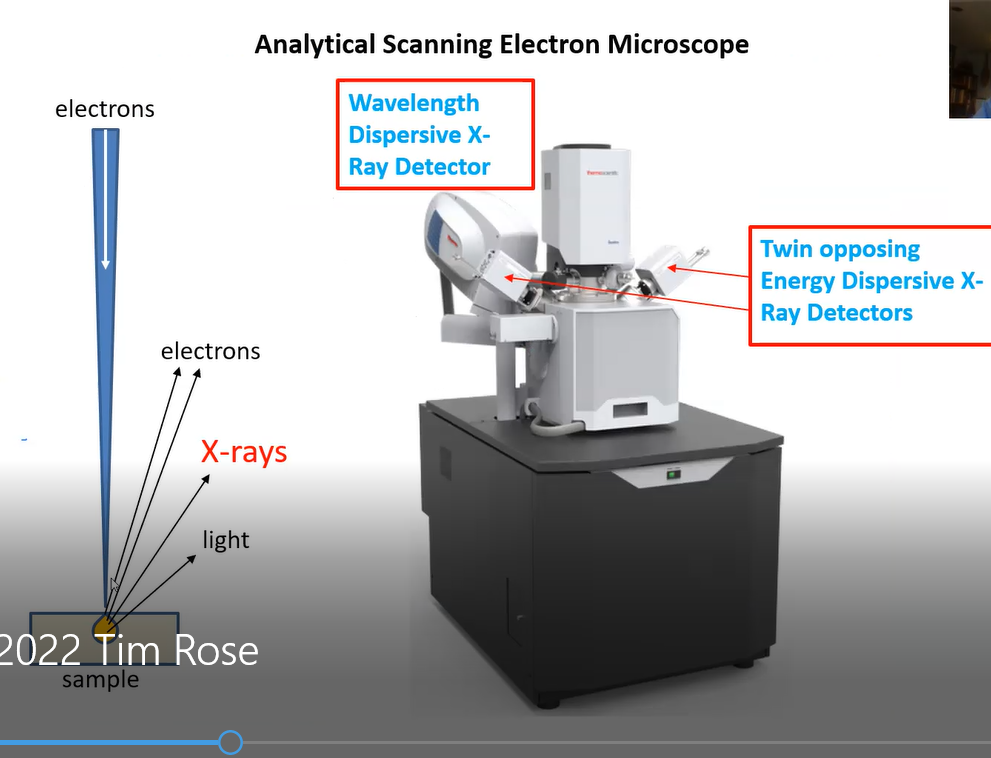
Tim then used the Mineral Sciences Department’s analytical scanning electron microscope (ASEM), shown below, and bombarded a very tiny portion of the specimen (which is not destroyed). The purpose of this test is to detect x-rays whose energy level or wave length is characteristic of and identifies which elements are present. The impact of the incoming electron beam, Tim said, produced x-rays as illustrated below. Their energy level or wavelength disclosed the identity of the chemical element in the sample that was bombarded.
The electron image that emerged from the x-rays of the supposed chrome diopside sample shown below, revealed that it is not one mineral, but three minerals. The black, dark grey and light areas are, respectively, clinochlore, diopside, and chromium grossular garnet. So, the analysis done by Larrabee was not of one mineral but three and that explains why there was aluminum in the diopside analysis. It came from the garnet. But where did the chromium in Larrabee’s analysis come from? Tim’s analysis of the diopside showed none. It is just diopside not chrome diopside.
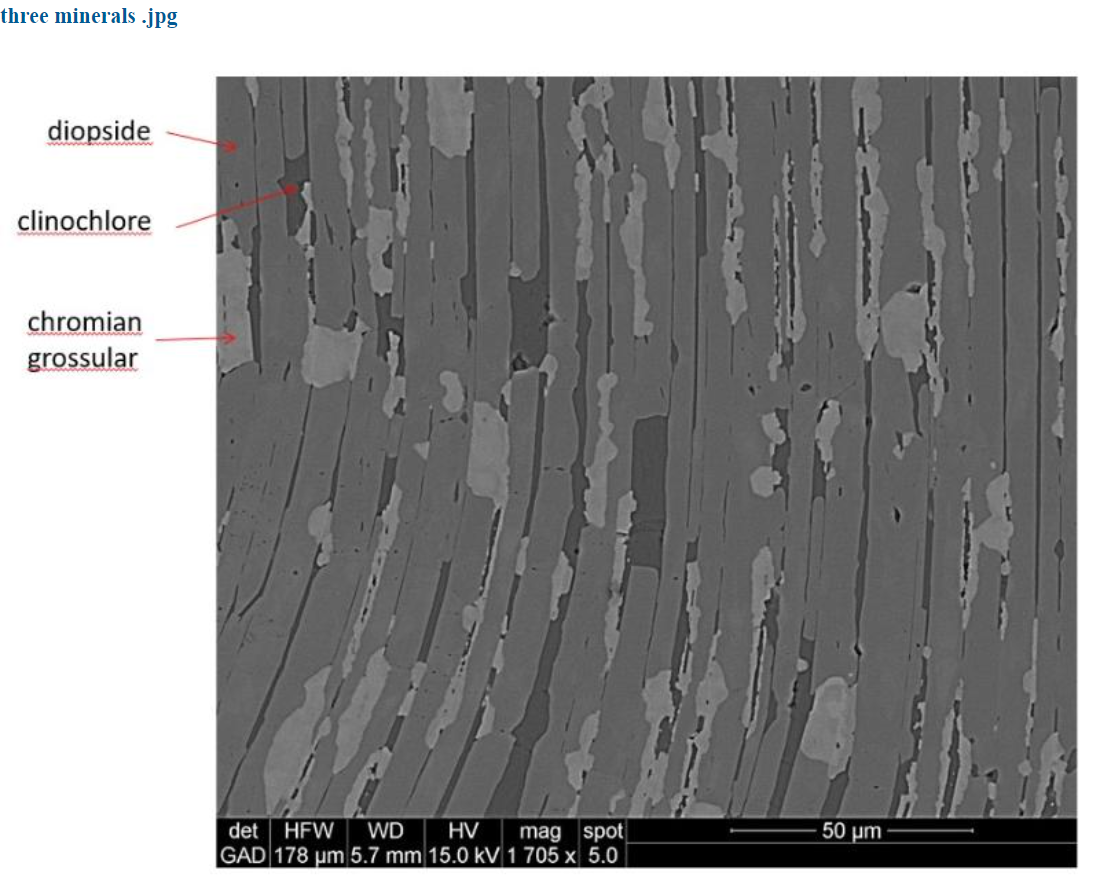
Tim’s further analyses found that the chromium was indeed present – not in the diopside, but in the grossular garnet! The x-ray spectrum of the garnet reveals peaks in the garnet from a small amount of chromium.
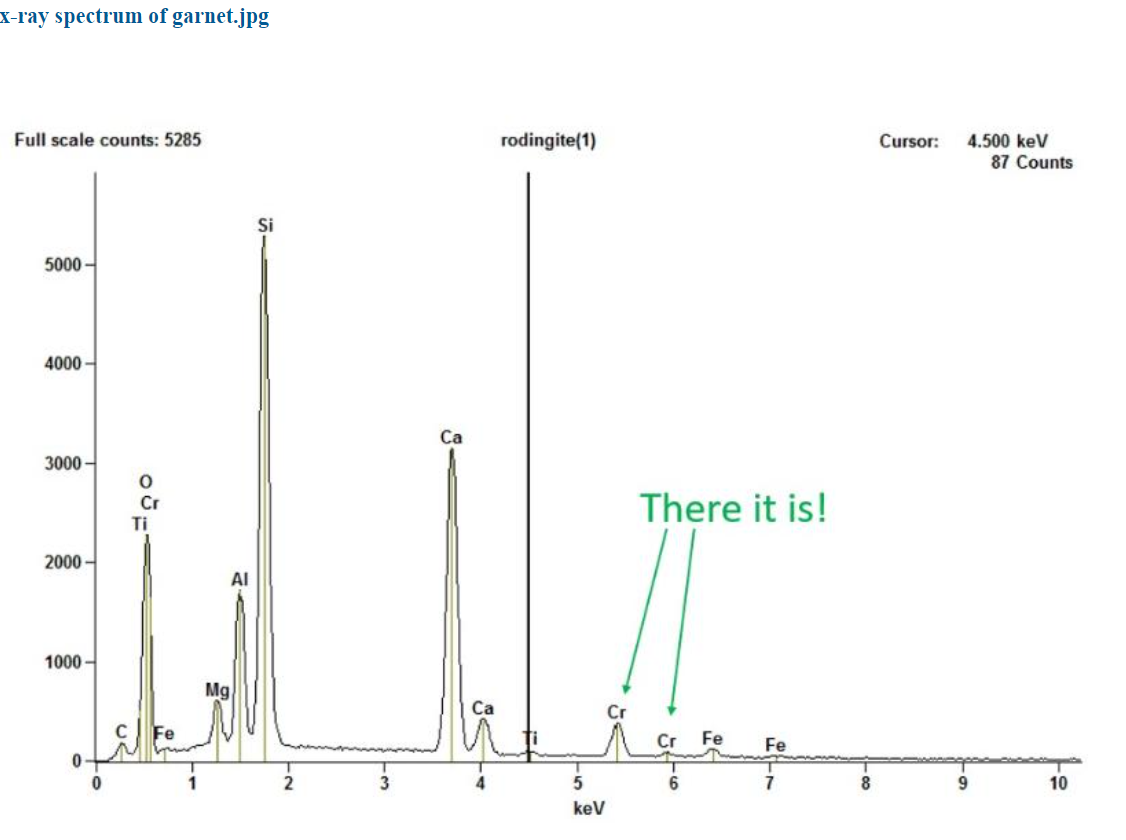
So now we know that what we used to think was a single mineral, chrome diopside, is actually three interlayered minerals which likely interact with light to give rise to the beautiful sheen that you see in the green areas in rodingite.
Tim concluded his exploration into rodingite and diopside by reviewing and highlighting his findings. (Viewers of Tim’s YouTube video, whose link is below, should watch for this clear summary statement.) He ended by saying: “That’s the story. You never really know what you have unless you look really close.”
Kenny Reynolds then opened the meeting for questions and further interesting explanations from Tim. This concluding portion of the evening’s session, like Tim’s findings and the quarry’s minerals, had many layers which readers can best enjoy by going to the YouTube recording which MSDC has posted to our YouTube channel.
Kenny and Cindy thanked Tim for his very interesting and wonderful presentation. The attendees signaled their pleasure and gratitude with sustained applause.

Here is the link for Tim’s Rose’ presentation (68 minutes): “Rodingite from Hunting Hill Quarry, Maryland”
